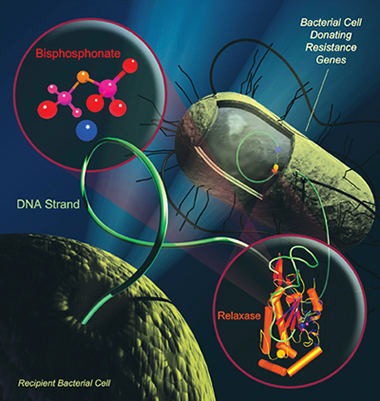
Bacteria spread antibiotic-resistance
via relaxase.
Illustration: Scott Lujan,
UNC-Chapel Hill |
A couple of old osteo drugs could
hold the key to fighting deadly antibiotic-resistant
bacteria like MRSA, according to a new study in the
July 13 Proceedings of the National Academy of Sciences.
A team of US researchers found
that clodronate and etidronate, first generation bisphosphonates,
block an enzyme in E coli cells that allows bacterial
'mating' and gene transfer. "The surprise was that they
also killed the cells carrying the resistance gene,"
says lead author Matt Redinbo, PhD, biochemist and biophysicist
at the University of North Carolina at Chapel Hill.
"Although, in retrospect, we shouldn't have been so
surprised, because normally if you screw with DNA movement
you would cause cell death."
The discovery opens up a slew of
possibilities in the fight against antibiotic-resistant
bacteria. "This may lead to the ability to selectively
kill antibiotic-resistant bacteria in patients, and
halt the spread of resistance in clinical settings,"
says Dr Redinbo. Although the study was done on lab
cultures, Dr Redinbo and his team have started an animal
study and are currently testing a wider variety of bacteria
to see if the bisphosphonates will work on them.
MUTANT
BACTERIA
Antibiotic resistance is cause enough for concern that
the CDC in the US has designated an entire program to
watch for it: the National Antimicrobial Resistance
Monitoring System (NARMS). More than 2 million people
a year get hospital-acquired infections in the US alone,
and 90,000 die. In Canada, an E coli outbreak
last September left 14 Ontarians severely ill, while
a simultaneous one in the US claimed the lives of three
people. Commonly found in human and farm animal intestines,
E coli spreads through fecal contamination and poor
hygiene.
"We worked with ampicillin-resistant
E coli ," says Dr Redinbo. "This bacteria uses the
same system to conjugate and spread as a wide variety
of infectious bacteria." The key component of that system
is an enzyme called relaxase.
When a person gets infected and
then treated with antibiotics, a sort of Darwinian natural
selection occurs. The weaker bacteria get killed off.
The ones that remain have a genetic mutation that allows
them to survive. They start spreading that mutation
quickly to other cells through conjugation, a sort of
mating dance mediated by relaxase.
During conjugation, two bacterial
cells, one of which has the antibiotic-resistance gene,
get close together and are joined by relaxase. Each
cell sends a single strand of its phosphate-rich DNA
to bind to a special site on the enzyme. Relaxase then
does a strand exchange, sending the resistance gene
to the weaker cell and helping the resistance spread
through the colony.
The researchers figured that the
conjugation phase would be a good time to target the
bacteria, hitting it where it hurts, so to speak, with
half its DNA hanging out. So they took a closer look
at relaxase. "One of my graduate students, Scott Lujan,
solved the problem," says a proud Dr Redinbo. "He looked
at the crystal structure of the enzyme and predicted
that its weakest point would be where it had to accommodate
the two DNA strands." Given the high phosphate content
of DNA, they suspected that another phosphate-rich compound
could block the dual binding site, hence the bisphosphonates.
"Etidronate and clodronate are
typically prescribed for bone loss," says Dr Redinbo.
"They've been replaced by newer generation bisphosphonates
in clinical use. We tested other bisphosphonates that
were easier to obtain — clodronate is not commercially
available in the US — but they didn't work as well."
Both drugs can have serious side
effects, ranging from stomach cramping to birth defects,
but Dr Redinbo believes that where there's a will, there's
a way. "A possible use is with Staph infections or skin
related infections where dosage can be tightly controlled,
or perhaps associated with a catheter for a more targeted
drug delivery," he suggests.
AS
SEEN ON TV
The relatively obscure biochem discovery has already
hit the small screen. "This morning, I was on the CBS
Early show," says Dr Redinbo.
The scientist quickly discovered
that his new-found celebrity comes with a cost, when
his phone started ringing off the hook. "I've had calls
from people saying 'I've got MRSA, how can I use this
drug to stop the infection?'" he says. "I'm not a clinician
and the research is still in its early stages, but we're
working on translating this work into a mammalian system."
Dr Redinbo and his team have applied
for a patent for their discovery and are currently testing
out the drugs with other bacteria. "This relaxase system
is found in Staphylococcus strains, Acinetobacter
strains and other common bacteria," says Dr Redinbo.
"These strains cause really prevalent infections, so
the hope is that these drugs will either help existing
antibiotics or offer a new treatment for antibiotic-resistant
bacteria.
|